How a well-meaning U.S. government database fuels dangerous vaccine misinformation
Editor’s Note: As of February 2024 the Misinformation Monitor is now Reality Check, a weekly newsletter on misinformation and media online. Learn more and subscribe here on Substack.
Written by Melissa Goldin, John Gregory, and Kendrick McDonald
Additional reporting by Chandler Kidd, Chine Labbé, Bron Maher, Virginia Padovese, and Marie Richter
How a well-meaning U.S. government database fuels dangerous vaccine misinformation
On April 30, 2021, the website Natural News — which NewsGuard has rated Red, meaning generally unreliable — published a story reporting the death of a 2-year-old who in late February had received the second dose of a Pfizer-BioNTech COVID-19 vaccine during the companies’ clinical trials for children. The only problem? Children under 5 did not begin receiving shots until April, according to a press release on the Pfizer website.
Natural News picked up the false claim from a familiar source of pandemic misinformation: Red-rated website Great Game India, which in January 2020 also spread a different falsehood that the COVID-19 virus was stolen from a Canadian lab.
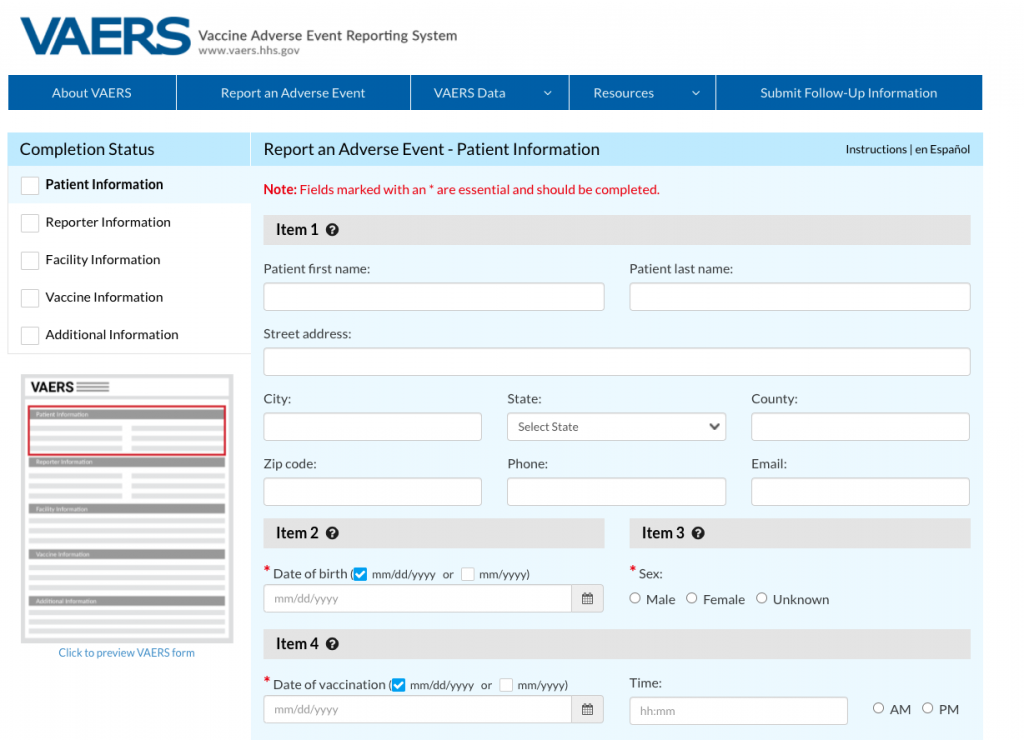
The single source of evidence cited by both websites was the Vaccine Adverse Event Reporting System, or VAERS, a database jointly run for 31 years by the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA). Its purpose: to be “a national early warning system to detect possible safety problems in U.S.-licensed vaccines,” according to its website.
It is true that a report was submitted to VAERS on March 5, 2021, which stated that a two-year-old from Virginia received a COVID-19 vaccine on Feb. 25, experienced the onset of side effects on March 1, and died two days later. The report also claimed that the toddler had been hospitalized for 17 days, which Great Game India interpreted to mean that “the baby was perhaps sick from the first shot. But someone administered the second shot anyway.”
Great Game India’s article also noted that Pfizer’s “own promotion says the vaccination trials were for children from age 5 to 11” and asked, “How come a 2 year old baby got vaccinated?”
The answer is that the incident never happened. CDC spokesperson Kristen Nordlund told USA Today, for an article fact-checking the Natural News story, that the adverse event report was “completely made up” and the CDC took the rare step of removing it from the VAERS system.
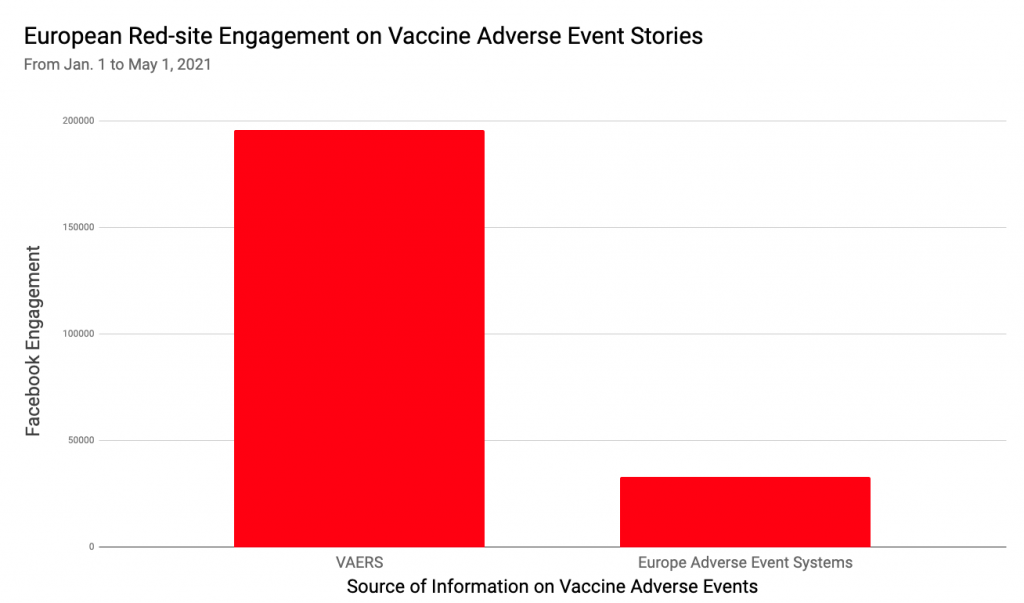
This is far from the only time that anti-vaccine advocates have used VAERS data to claim, falsely, that COVID-19 vaccines can or have caused death, infertility, or other side effects. Using data from NewsWhip, a social media intelligence company, NewsGuard has found that Red-rated sources like Natural News and Great Game India account for over 80 percent of Facebook engagement on stories that prominently cite VAERS.
As explained below, VAERS was established in 1990 as part of federal legislation aimed at requiring health care providers to report incidents of adverse effects related to vaccines. Although the reports have never been vetted before being included in the VAERS database, the hope was that reports were rooted in reality or at least in good faith assumptions that an illness or other injury may have been related to a vaccine. But that was before the modern internet, which allows anyone to report anything to VAERS for instant public posting.

Noisy by Design: A Platform for Vaccine Information (and Misinformation)
VAERS is a noisy system by design. It collects unverified reports of any adverse health events reported to have happened following vaccination. The database includes reports based entirely on hearsay, or lacking a plausible link to a vaccine, such as someone dying in a car accident on their drive home from getting a vaccine. Although vaccine manufacturers are required to submit reports, anyone can submit a report to VAERS, without providing a name or contact information.
A system like VAERS with an abundance of information onto which everyone can project their concerns or agendas has all the potential benefits — and pitfalls — of a Facebook-like service for those focused on vaccines. And during the largest vaccination effort in history, it serves as an early warning system for real adverse effects, while to a small but vocal set of activists, it enables the sounding of a false alarm at a much louder volume.
The exploitation of VAERS by the anti-vaccine movement during the COVID-19 vaccine rollout is not a surprise to Dr. Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia and a member of the FDA’s vaccine advisory committee.
“The anti-vaccine people will always say, ‘Look at all these deaths, look at all the damage that these vaccines are doing, they’ll always do that, and there will always be a group of people who believe them, and the only way that’s going to change if we all get to move to a planet that’s dominated by reason and logic,” Offit said.
Nevertheless, Offit and other public health officials see the system’s vulnerability to manipulation as a necessary risk, and suggest that the scope and transparency of VAERS is a testament to how seriously the government takes vaccine safety and the potential risks.
A ‘Hypothesis Generator’: Accessibility Makes VAERS Effective, but Vulnerable to the ‘Incredible Hulk’
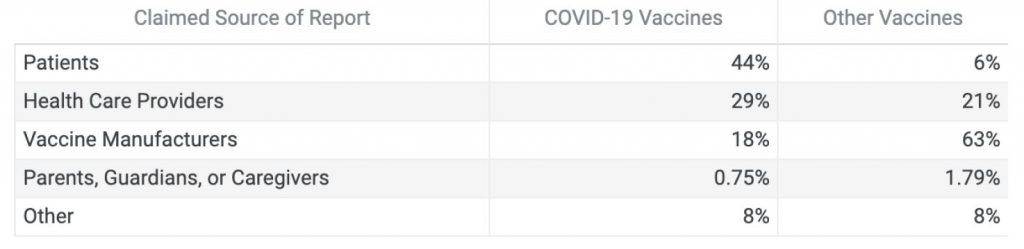
In the first six months of the COVID-19 vaccine rollout, the biggest single source of VAERS reports related to the COVID-19 vaccine has been the general public. For other vaccines, manufacturers have typically accounted for the majority of reports.
Claimed Sources of VAERS Reports from Dec. 14, 2020 to May 10, 2021
This trend toward crowd-sourcing is not all bad. Because anyone can report an incident to VAERS, the system can detect rare and serious adverse events quickly. Identifying the rare risk of blood clots with low platelets following the use of Johnson & Johnson’s one-shot COVID-19 vaccine is one example where VAERS worked as intended, researchers said.
“The clinical trials were large, but they weren’t large enough to get a handle on an event that might be one-in-a-million,” said Susan Ellenberg, professor of biostatistics, medical ethics and health policy at the University of Pennsylvania’s Perelman School of Medicine. “Once you start vaccinating multiple millions of people, you may start to see things, and that was unusual enough that it got the attention of the people monitoring these data.”
Having such a broad database allows VAERS to act as a “hypothesis generator,” Dr. Tom Shimabukuro, deputy director of the CDC’s Immunization Safety Office, told NewsGuard in a phone interview. Once an unexpected pattern or safety concern is picked up, government health agencies can test that hypothesis with the help of vaccine monitoring systems, such as the Vaccine Safety Datalink, where the CDC has access to the electronic health data of 12 million people from nine large health care organizations.
However, the accessibility and transparency of VAERS also makes the system vulnerable to fake reports, including through a large, coordinated effort by anti-vaccine organizations, if they chose to do so.
“If people did want to get together in an organized way and flood the system with reports, I mean, that could happen,” the CDC’s Shimabukuro said. “We think that’s rare…. We have to strike a balance between making the system accessible and making sure we’re not getting flat out fraudulent reports.”
Fake reports have been identified in the past. In one infamous example, anesthesiologist James Laidler demonstrated the potential for abuse of VAERS by submitting a report in 2004, claiming that the flu vaccine turned him into the Incredible Hulk. The report was removed from VAERS after the CDC contacted Laidler and asked for his permission to delete it.
The CDC says that it follows up on every death reported to VAERS by requesting medical records, autopsy reports, and death certificates. “It was during that process that CDC discovered that the report [about the two-year-old’s death] submitted to VAERS was false,” Nordlund said in an email to NewsGuard. She declined to explain exactly how the CDC determined the report in this case was false.
‘Meaningless to the General Public’: VAERS Discloses Limitations — But Alternative Platforms Hosting VAERS Data Do Not
On the VAERS website, there is no shortage of warnings about how data should not be interpreted. Pages titled About VAERS, A Guide to Interpreting VAERS Data, and VAERS Data all include clear statements that a report does not imply a vaccine caused the adverse event. A Frequently Asked Questions page lists the strengths and limitations of VAERS, explaining that VAERS data “cannot determine if the vaccine caused the reported adverse event” and acknowledging that this “has caused confusion in the publicly available data… specifically regarding the number of reported deaths.” The list of limitations also states that VAERS data “sometimes contains errors” and “cannot be used to determine rates of adverse events,” among other points.
And even for those who do not look at these pages, accessing VAERS data requires a reader at least to glance at a disclaimer explaining the limitations of the database and then to click a button labeled “I Agree” before searching individual reports.
With the rollout of COVID-19 vaccines, the CDC has provided additional analysis of VAERS reports on its main website, stating, “A review of available clinical information, including death certificates, autopsy, and medical records has not established a causal link to COVID-19 vaccines.”
However, alternative platforms exist where anyone can access VAERS data and bypass the CDC’s clear explanation of its many limitations. One, MedAlerts.org, is run by an anti-vaccine group called the National Vaccine Information Center, whose own website NewsGuard found to have repeatedly published false health claims.
Another, OpenVAERS.org, does not identify who is behind the site, although a site representative told NewsGuard in an April 2021 email that is owned by “a small group of parents with vaccine injured children.” The site lists raw numbers of deaths and hospitalizations attributed to COVID-19 vaccinations, without the caveat that those reports have not been verified to ascertain that the event that was reported actually happened, let alone that it was related to a vaccine.
Dr. Offit, of the Children’s Hospital of Philadelphia and the FDA’s vaccine advisory committee, told NewsGuard that while the system can be helpful to medical practitioners and public health officials, he believes that its value to the general public is questionable. “I think VAERS is meaningless to the general public because people can’t look at that and make any comments about whether or not those associations are real,” he said.
The Facebook Effect: Distorted VAERS Data on Social Media
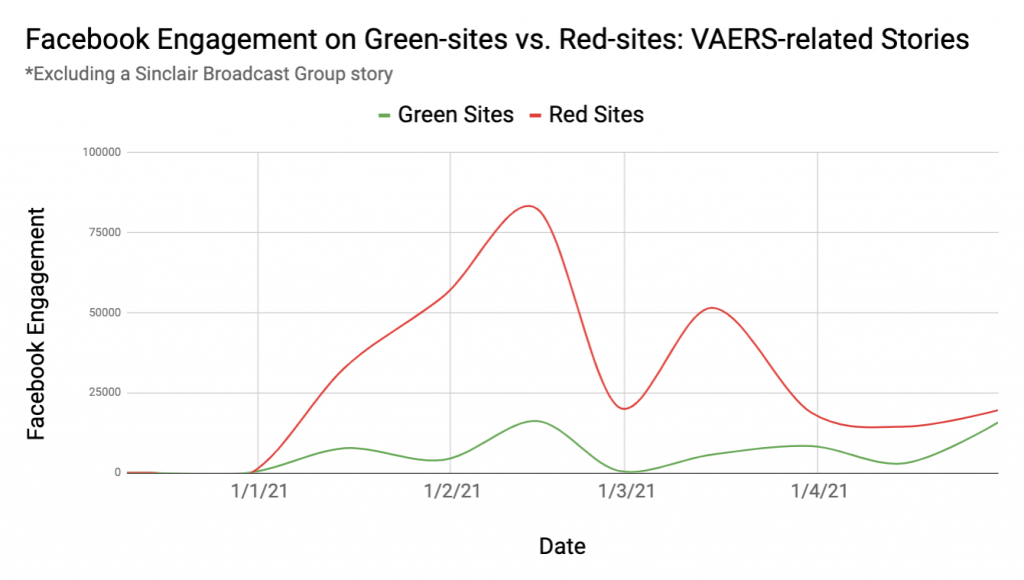
Between Dec. 11, 2020, when the FDA first authorized a COVID-19 vaccine, and May 2021, articles that prominently mentioned VAERS (meaning VAERS was named in the headline or article summary, a metadata property of the story) received over 1.1 million interactions on Facebook — such as likes, comments, or shares — according to data from NewsWhip, a social media intelligence company.
Notably, about two-thirds of that engagement came from a single story mentioning VAERS, about a Utah woman who died in February after receiving the second vaccine dose. The story was published on the websites of at least 58 Sinclair Broadcast-owned local television stations. Many of the articles were later updated to note that the Utah Medical Examiner’s Office released a statement that “There is no evidence COVID-19 vaccines have caused any deaths in Utah.”
Excluding that story as an outlier, NewsGuard found that over 80 percent of Facebook engagement on VAERS-related stories came from Red-rated websites, including notable sources of vaccine misinformation such as Children’s Health Defense, a group run by Robert F. Kennedy Jr.
Some articles that distort VAERS data state, declaratively, that vaccines were the cause of the reported side effects. For example, personal trainer and life coach Christian Elliot stated on his Red-rated website DeconstructingConventional.com that “at the time of this writing VAERS reports over 2,200 deaths from the current covid vaccines, as well as close to 60,000 adverse reactions.”
Many articles reviewed by NewsGuard do include some acknowledgement that VAERS publishes unconfirmed data, or are careful to report on deaths “following” a vaccine, rather than explicitly stating that they were “caused by” the vaccine.
For example, Children’s Health Defense stated in a February 2021 article that “VAERS is the primary mechanism for reporting adverse vaccine reactions in the U.S. Reports submitted to VAERS require further investigation before confirmation can be made that an adverse event was linked to a vaccine.”
However, these warnings, where they exist, are often overshadowed by misleading headlines, a long and detailed repetition of VAERS data, fearful or speculative questions about what it might mean, and advocacy against the well-documented safety and efficacy of vaccines.
For example, anti-vaccine advocates frequently claim that VAERS data is vastly underreported, and extrapolate to claim that far greater numbers of reactions are actually occurring. Joseph Mercola, who received a warning letter from the FDA in February about marketing false COVID-19 cures, stated in an article that month, “According to a U.S. Department of Health and Human Services [HHS] study, fewer than 1% of vaccine adverse events are ever reported to VAERS.” He added that “there may, in reality, be over 1 MILLION COVID vaccine injuries, since 99% typically go unreported.”
It is true that a 2011 report by health insurance company Harvard Pilgrim Health Care that was submitted to HHS asserted that “fewer than 1% of vaccine adverse events are reported,” without citing a specific source for that figure. However, especially given the scope of the COVID vaccination effort and the attendant publicity, it is misleading to claim that all vaccine adverse events are equally underrepresented in VAERS, or that the 1 percent figure could be used to calculate the “real” number of adverse events.
“One potential explanation for low reporting of mild events might be that many are expected, and therefore people don’t feel the need to report them,” the CDC’s Immunization Safety Office told Agence France-Presse in December 2020. “As an example, sore arms are an expected event after certain vaccines so people may decide not to report this event to VAERS.”
Shaydanay Urbani, Partnerships and Programs Manager for First Draft News, a nonprofit that addresses mis- and disinformation, told NewsGuard that even if articles quote from the VAERS website to state that the data is unverified — which Children’s Health Defense does in its reporting — this disclosure does little to help readers looking to make an informed decision about the safety of COVID-19 vaccines.
“In my opinion, it’s not enough to quote the disclaimer, if you’re using the [VAERS] data, because at the end of the day, if what your article is doing is quoting data, drawing a conclusion from it, but then saying the data isn’t verified, then how is that reporting?” Urbani said. “How is that providing reliable information to the public? What role have you provided in helping people understand what’s going on with the vaccine?”
Liability, Accountability, Misrepresentation: The History of VAERS
VAERS was established in 1990 by the CDC and FDA in response to the 1986 National Childhood Vaccine Injury Act (NCVIA), which among other provisions requires health care providers to report adverse effects that may have been caused by vaccines.
Prior to VAERS and the NCVIA, the number of lawsuits against vaccine manufacturers and health care providers rose dramatically in the 1970s and 1980s. This meant that manufacturers were forced to compensate individuals and families for alleged vaccine injuries, such as from the diphtheria-pertussis-tetanus (DPT) vaccine, even if there was no scientific evidence to support the claims.
Consequently, many pharmaceutical companies ceased producing vaccines as potential liability, legal fees, and compensation costs mounted. For example, only one U.S. company manufactured the DPT vaccine by the end of 1984. The U.S. Congress responded by passing the NCVIA, in an effort to reduce potential liability and address public health concerns.
Besides VAERS’ role in identifying the rare risk of blood clots that can occur following the use of Johnson & Johnson COVID-19 vaccine, the system has helped scientists identify risks associated with other vaccines.
In October 1999, the vaccine RotaShield, which prevents rotavirus gastroenteritis (severe diarrhea and dehydration) was voluntarily taken off the U.S. market by its manufacturer following reports of adverse events on VAERS. The move came a little more than a year following its U.S. approval in August 1998, after some infants developed intussusception, a rare type of bowel obstruction, after receiving the vaccine.
“I will never forget, I was director of the United States immunization program when we broke out our first rotavirus vaccine,” Dr. Walter Orenstein, associate director of Emory Vaccine Center and professor of infectious diseases at Emory School of Medicine, told NewsGuard. “The head of my immunization services division came up to me and said, there’s a cluster of valve blockage called intussusception, about a week afterwards. And that led to a signal — hey, is this real or not?”
However, adverse events have always been vulnerable to misrepresentation, such as in the case of LYMErix, a vaccine that prevents Lyme disease.
The vaccine was approved by the FDA in December 1998 and recommended by the U.S. Advisory Health Committee (ACIP) in 1999 for people living in areas with a high-risk of Lyme disease or who engaged in activities where they were frequently exposed to ticks. LYMErix received considerable media coverage, little of which discussed the vaccine’s potential risks.
However, reports of adverse reactions allegedly caused by LYMErix began appearing on VAERS about a year after it was licensed by the FDA. These reports received wide media coverage, and people claiming to have been injured by the vaccine were branded as “vaccine victims.” In December 1999, the Philadelphia law firm Sheller, Ludwig & Bailey filed a class action lawsuit against LYMErix’s manufacturer.
Although subsequent studies done by the FDA and the vaccine’s manufacturer did not find any evidence that the vaccine was causing harm, scientists did discover that some people may be genetically predisposed to having a greater risk of developing chronic, treatment-resistant arthritis after receiving LYMErix.
The FDA formed an advisory panel on LYMErix in January 2001, amid pending lawsuits and concern from the public and the media. The panel concluded that the benefits of LYMErix outweighed the risks. Nevertheless, while the vaccine remained available for public use, sales fell dramatically.
In February 2002, LYMErix’s manufacturer voluntarily withdrew the vaccine from the U.S. market. The company also settled several class action suits, stating that while it still believed that LYMErix was safe, the company settled due to economic concerns. The FDA did not revoke LYMErix’s license, but there is currently no Lyme disease vaccine available to the public.
Checks and Balances: European Systems Provide Different Safeguards
As the global vaccine rollout continues, or in some cases gets underway, other countries are likewise relying on their own adverse event monitoring systems, with some similarities and some differences to VAERS.
Like VAERS, adverse event reports in France, Italy, Germany, and the U.K. can be submitted by anyone. However, of those countries, only Germany accepts anonymous reports. (The Paul-Ehrlich-Institut, Germany’s federal institute for vaccines and biomedicines, nevertheless urges people to provide all available information.)
The U.K. system, called the Yellow Card Scheme, was established in 1964 in the wake of the thalidomide scandal, a drug prescribed to pregnant women that resulted in thousands of birth defects. For COVID-19 vaccines, the U.K. has published summaries of Yellow Card reports, rather than raw data.
The same is true of systems in France, Italy, and Germany, in contrast to the searchable data tables displayed in VAERS.
In a phone interview with NewsGuard, Mehdi Benkebil, deputy director of the surveillance direction at ANSM, France’s National Agency for the Safety of Medicines and Health Products, said, “We did not choose to publish the raw data, but to instead publish analyzed information, from a scientific and medical standpoint.”
Anna Rosa Marra, Director of the Pharmacovigilance at Italy’s Medicines Agency (AIFA), expressed a similar sentiment, telling NewsGuard, “The policy adopted by AIFA is a policy of transparency supported by scientific data. In order to provide data to the public, both general and specialist, we have chosen to publish monthly reports with analysis of the data relating to the vaccines in use in our territory, giving the possibility to access information in an aggregate manner, but without hiding anything.”
In France, all adverse event reports are reviewed at a drug safety center, either by a doctor or pharmacist, and patients are contacted in cases of serious events. The raw data is not made public by ANSM. Benkebil said that while he has not heard of any cases of false reports related to COVID-19 vaccines, ANSM would be prepared for them. “Typically, it’s something we would easily identify… That’s what our experts are for,” he said.
Marra said that she has not worried about false reports in Italy in part because “There is an increase in reports from health professionals with COVID vaccines compared to other vaccines or other drugs according to the usual trends.” About 81 percent of the COVID-19 AIFA reports have come from health professionals, she told NewsGuard. AIFA reports cannot be anonymous.
This is the opposite trend seen in VAERS reports, where patients make up a greater portion of submitted reports than normal.
Local reports from European Union countries like France, Germany, and Italy are fed to EudraVigilance, an E.U. database managed by the European Medicines Agency (EMA), which does have raw data for users to search through. The homepage, though, includes three bullet points of “Key Information,” including a statement that “Information on suspected side effects should not be interpreted as meaning that the medicine or the active substance causes the observed effect or is unsafe to use.” Individuals cannot make reports directly to EudraVigilance; they must go through their country’s medical agency, such as ANSM or AIFA.
Marra told NewsGuard the system is like a pyramid, with EudraVigilance at the top and local medical professionals taking the first steps to investigate reports at the bottom.
“The Italian national pharmacovigilance network is coordinated by AIFA and AIFA in turn coordinates the regional pharmacovigilance centers,” she said. “In each health facility there is a local pharmacovigilance manager. The local pharmacovigilance managers, who are all registered and authorized by AIFA, are the ones who validate the reports.”
Of course, the populations in these countries, and therefore the number of adverse event reports, is much smaller than in the U.S. In addition to differences in the presentation of data, this may be another reason why, according to NewsGuard’s research, European Red-rated sites spreading vaccine misinformation reference local adverse event reporting systems less frequently than they reference VAERS.
Indeed, many Red-rated European websites with vaccine misinformation often cite VAERS data — a foreign source — thus raising fears and doubts about vaccine safety in their own countries.
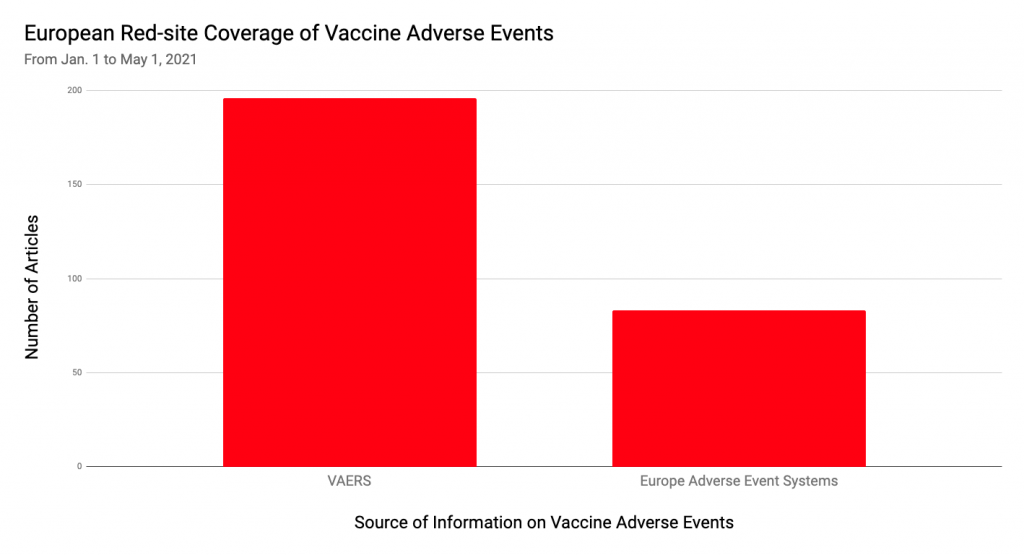
For example, in April 2021, French Red-rated site Planetes360.fr published an article based on VAERS data that claimed that: “736 persons died within 48 hours after receiving the COVID vaccine.”
A January 2021 article on Russian propaganda site De.News-Front.info claimed in German: “According to the U.S. federal Vaccine Adverse Event Reporting System database, 55 people died within days of vaccination. It is worth noting that patients who had already died were injected with both the notorious Pfizer vaccine and the Moderna drug.”
Also in January 2021, Italian Red-rated site Renovatio21.com published an article, headlined “Vaccine, 55 deaths in the US (according to official document),” that claimed: “Fifty-five people died in the United States following the administration of the Pfizer and Moderna vaccine. This was revealed by the latest report on drug vigilance produced by VAERS.”
This is not to say that there is no vaccine misinformation referencing other adverse event reporting systems. Summaries from the U.K.’s Yellow Card Scheme have been cited by NewsGuard Red-rated sites that frequently treat Yellow Card reports as statements of fact. Moreover, those that acknowledge that Yellow Cards are user-submitted may nonetheless argue that reports of adverse events following vaccination indicate a trend.
For example, a March 2021 article, published by Red-rated site VernonColeman.com, stated: “According to the latest yellow card reports from the MHRA in the UK, a total of 20 women have now had miscarriages and lost their unborn babies after having one of the mRNA vaccines. You might argue that some or all of those women would have lost their babies without the vaccine. But you don’t know that. No one knows.”
‘Those of Us Who Work in Science Can Tear Our Hair Out’
VAERS has been used by scientists as an investigative tool to help their research and by anti-vaccine organizations to further their agenda by using unverified reports that often lead to misinformation. Both groups, despite the differences in how they apply VAERS to their work, tend to agree that the benefits of VAERS are worth the trade-offs and that there is value in the system’s transparency.
Ellenberg, the University of Pennsylvania professor, believes that a transparent system that has the potential to be misused is better than leaving people in the dark.
“With public access, there’s going to be misinterpretation of the data there,” she said. “But without public access, I think it’s worse. Because then you just have people imagining what’s there. And that can really be worse.”
Views on VAERS are similar in the anti-vaccine world, even though the system is not often used for its intended purpose among these groups.
Barbara Loe Fisher, co-founder and president of the National Vaccine Information Center, told NewsGuard that VAERS allows people who have received vaccines, or their families, to submit reports “if a vaccine provider fails to report a serious event” and check whether it has been received. She also noted that “VAERS transparency also ensures that scientists have open access to the VAERS database to use as a research tool.”
The question of whether the benefits of VAERS actually outweigh the risks remains a matter of debate. However, Professor Ellenberg said she believes that educating the public about the system and its purpose is the best way to counteract any unintended harm.
“Those of us who work in science can tear our hair out about the way people misinterpret things,” she said. “And all we can do is to continue to try to educate people.”
And, of course, now that the VAERS data in raw form has been public for so long, cries of a deep state conspiracy would certainly follow any move to shut the data off.
Send us ideas or questions.
Download NewsGuard
Install our browser extension to see NewsGuard’s shields in your search engine results and Facebook, Twitter, and LinkedIn feeds on your desktop browser. Download our new mobile app, available for iOS and Android.